Subcutaneous Defibrillator (S-ICD)
The S-ICD System is the world’s first ICD that provides defibrillation therapy without touching the heart. It is a completely subcutaneous defibrillator that does not require leads in the heart, leaving the vasculature untouched. It can be placed strictly by anatomical landmarks, removing the need for fluoroscopy at implant. It is an alternative to the conventional transvenous implantable cardiac defibrillator and was approved by the FDA in late 2012.
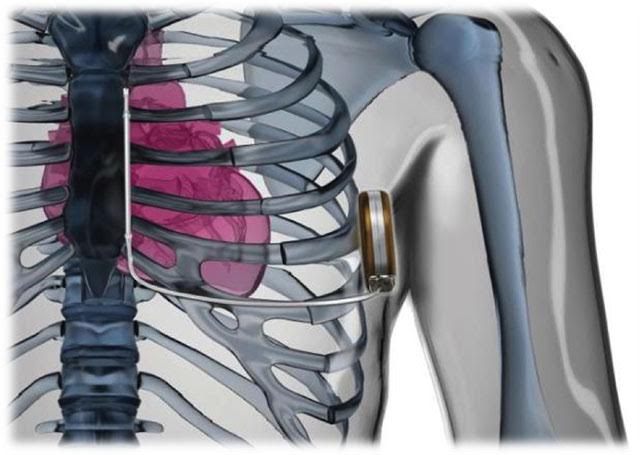
The S-ICD System is intended to provide defibrillation therapy for the treatment of life-threatening ventricular tachyarrhythmias in patients who do not have symptomatic bradycardia. It can still be an option for patients who already have a preexisting functional transvenous bipolar pacemaker.
VIDEO: How a subcutaneous ICD is implanted
The S-ICD provides effective defibrillation for ventricular tachyarrhythmias , significantly reduces the risk of vascular injury at the time of implant, has a low risk of systemic infection given no hardware in the venous system, preserves venous access (a high priority for some patients including those with end-stage renal disease who are on or are preparing for hemodialysis), avoids risks associated with endovascular lead extraction if future lead fracture or infection, and again requires no fluoroscopy at the time of implant.
Your electrophysiologist will able to discuss this option more in-depth with you to determine if you are a good candidate and proceed with in-office required preoperative 3-lead surface electrocardiogram screening to ensure that you are a reasonable candidate for this therapy (given the appropriateness of surface signals).




 Silver Spring Office
Silver Spring Office  DC Office (at Providence Hospital)
DC Office (at Providence Hospital)  Hagerstown Office
Hagerstown Office